
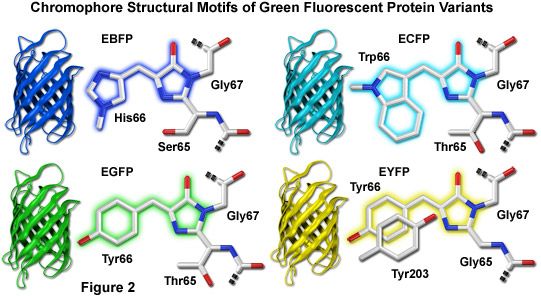
How did the authors make the GFP plasmid? The link below gives more information about why these types of vectors are useful for transformation into E. This promoter is widely used for bacterial transformation because it gives very high expression of the desired protein. This vector uses the T7 promoter to drive expression of GFP. The authors chose to use the pET3a plasmid for transforming bacteria with GFP. Cells were photographed during irradiation with a hand-held long-wave UV source. The bacteria on the right side of the figure have the GFP expression plasmid. victoria, chromophore formation is not species-specific and occurs either through the use of ubiquitous cellular components or by autocatalysis.įig. Because this fluorescence requires no additional gene products from A. Here, we show that GFP expressed in prokaryotic and eukaryotic cells is capable of producing a strong green fluorescence when excited by blue light. victoria were needed for the production of the fluorescent protein, we tested GFP fluorescence in heterologous systems. To determine whether additional factors from A. The mechanisms that produce the dehydrotyrosine and cyclize the polypeptide to form the chromophore are unknown. The GFP chromophore is derived from the primary amino acid sequence through the cyclization of serinedehydrotyrosine-glycine within this hexapeptide (7). Although the intact protein is needed for fluorescence, the same absorption spectral properties found in the denatured protein are found in a hexapeptide that starts at amino acid 64 (6, 7). This fluorescence is very stable, and virtually no photobleaching is observed (5). Purified GFP, a protein of 238 amino acids (3), absorbs blue light (maximally at 395 nm with a minor peak at 470 nm) and emits green light (peak emission at 509 nm with a shoulder at 540 nm) (2, 4). victoria that derives its excitation energy from aequorin (2), the green fluorescent protein (GFP). This light is the result of a second protein in A.
Although activation of aequorin in vitro or in heterologous cells produces blue light, the jellyfish produces green light. Light is produced by the bioluminescent jellyfish Aequorea victoria when calcium binds to the photoprotein aequorin (1). Even 20 years after its initial publication, Chalfie et al.'s paper endures as an example of a scientific milestone. The work of Chalfie and two of his contemporaries, Osamu Shimomura and Roger Tsien, earned them a Nobel Prize in Chemistry and has enabled great advances in understanding cancer, HIV, and other medical conditions. In the 1990s, Chalfie took advantage of GFP's bioluminescence and developed a method to insert its ability to glow into other proteins. In the 1960s, Martin Chalfie extracted Green Fluorescent Protein (GFP), a protein that emits a green glow, from the jellyfish Aequorea victoria. Although it is not the only protein producing bioluminescence (the ability of some organisms to produce their own light), GFP is unique because it does not require any additional chemicals or enzymes to form properly. Modern biomedical researchers have overcome this with a unique solution: make the proteins glow. Scientists' ability to track the activity of proteins has long been limited by our ability to see the proteins we're tracking.


 0 kommentar(er)
0 kommentar(er)
